We are accelerating a pipeline of potentially best-in-class oncology therapies to fight cancers with high unmet medical needs.
Program & Target
IND-Enabling
Phase 1
Phase 2
Partner
Rights
AVZO-021
Cyclin-Dependent Kinase 2 (CDK2) inhibitorGlobal, excluding Greater China1
AVZO-021 is a potential best-in-class selective CDK2 inhibitor for HR+ breast cancer, and other tumors with elevated cyclin E expression, such as ovarian cancer.
View Mechanism of ActionAVZO-023
Cyclin-Dependent Kinase 4 (CDK4) inhibitorGlobal, excluding Greater China1
AVZO-023 is a highly potent, potential best-in-class selective CDK4 inhibitor for HR+ breast cancer and other tumors.
View Mechanism of ActionAVZO-1418
EGFR/HER3 Bispecific antibody‒drug conjugate (BsADC)Global, excluding Greater China1
AVZO-1418 is a potential best-in-class BsADC targeting EGFR and HER3. EGFR and HER3 are co-expressed in many solid tumors, including non-small cell lung cancer (NSCLC). In addition, HER3 overexpression is a known resistance mechanism to EGFR inhibition, making the therapeutic combination of targeting both EGFR and HER3 a rational BsADC approach.
View Mechanism of ActionVAC-103
Nectin4/TROP2 Bispecific antibody‒drug conjugate (BsADC)2Global, excluding Greater China1
VAC-103 is a potential best-in-class BsADC targeting Nectin4 and TROP2. Nectin4 and TROP2 are co-expressed in many solid tumors, including urothelial and breast cancers. VAC-103 is being evaluated as a potential new approach to overcome resistance to or limitations associated with the existing standard of care, including for patients with locally advanced or metastatic urothelial cancer.
View Mechanism of Action1Greater China includes People’s Republic of China, Hong Kong, Macau and Taiwan.
2Exclusive Option Agreement
Our Science
Our pipeline programs leverage distinct mechanisms of action, specifically designed to target validated pathways with the goal of advancing the treatment of solid tumors.
Our Programs
AVZO-021
We are developing a potential best-in-class selective CDK2 inhibitor, AVZO-021 (ARTS-021), for HR+ breast cancer, and other tumors which have elevated cyclin E expression such as ovarian cancer and small cell lung cancer.
AVZO-022
We are developing a potential best-in-class selective CDK4 inhibitor, AVZO-022 (ARTS-022), for HR+ breast cancer and other advanced solid tumors.
CDK2 and CDK4 Inhibitors
FDA-approved CDK4/6 inhibitors target the cyclin D1-CDK4/6-pRb axis, which is dysregulated in HR+ breast cancer. In addition, breast cancer cells are more reliant on CDK4 compared with CDK61. Despite the impact of approved CDK4/6 inhibitors in HR+ breast cancer, patients experience hematologic toxicity due to the key role of CDK6 in hematopoiesis2 and many progress due to resistance associated with CDK2 hyperactivation enabling cell cycle progression. Therefore, the combination of a selective CDK4 inhibitor and selective CDK2 inhibitor may achieve better outcomes for patients.
1
2
3
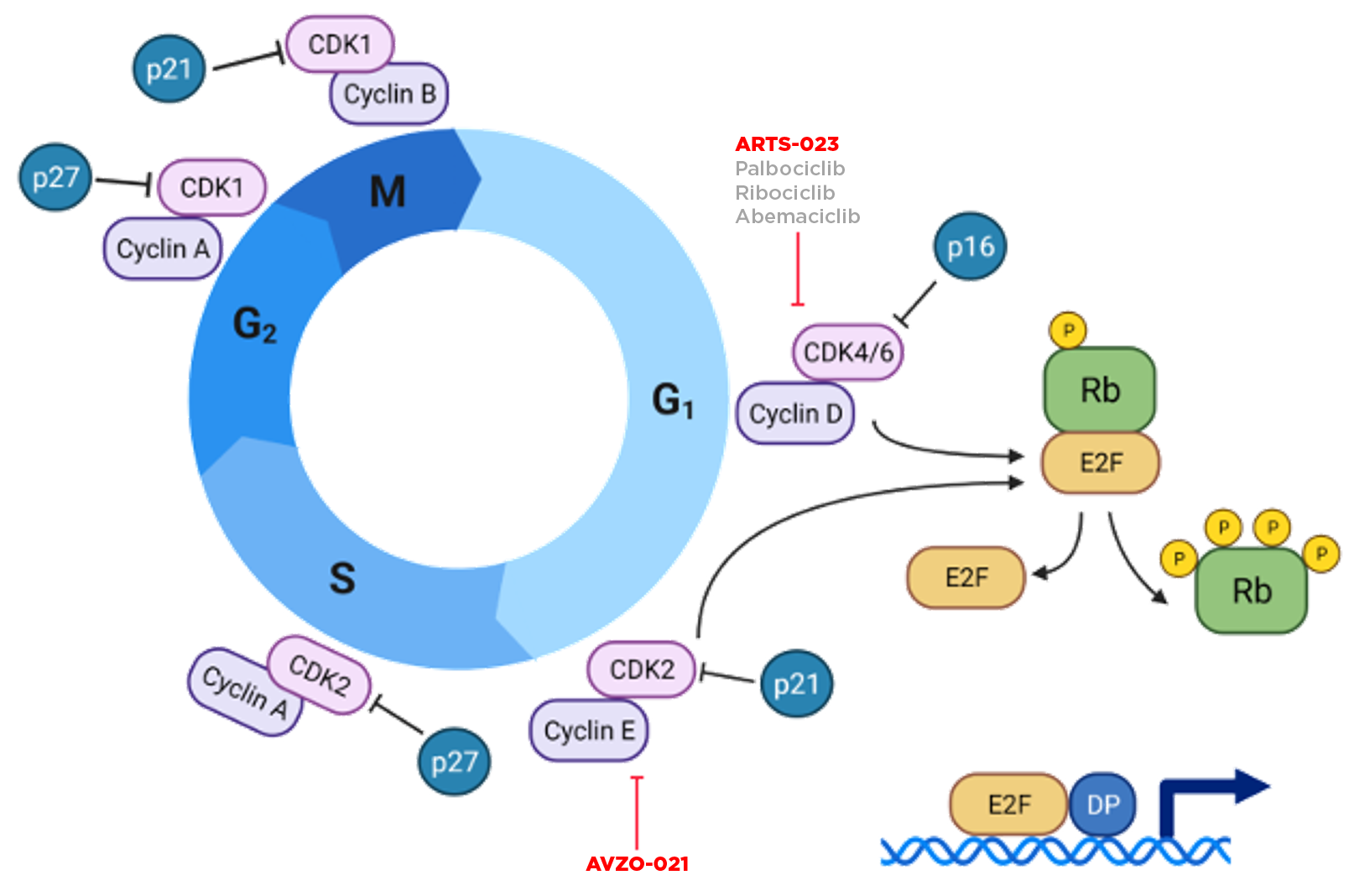
1
The cyclin D1-CDK4/6-pRB axis acts as a checkpoint during the cell cycle transition from cell growth (G1) to DNA synthesis (S). Its deregulation or overexpression induces abnormal cell proliferation.3,4
2
A key resistance mechanism to CDK4/6 agents is hyperactivation of CDK2 (through cyclin E elevation or other mechanisms).1
3
The combination of a selective CDK4 inhibitor with a selective CDK2 inhibitor may lead to more durable responses and disease control in HR+/HER2- breast cancer in the frontline and adjuvant settings by maximizing CDK4 target coverage and sparing CDK6-associated toxicity, while targeting a key mechanism of resistance.5,6
Note: Figured adapted from 1 Turner et al., Clin Oncology, 2019 2 Zhang Z, et al: Cancer Res (2022) 3 Bai et al., Cancer Bio & Med, 2017. 4 VanArsdale et al., Clinical Cancer Research, 2015. 5 Patel et al., Mol Cancer Research, 2018. 6 Al-Qasam et al., Nature Precision Oncology, 2022
Hyperactivation of CDK2 (through cyclin E elevation or other mechanisms) represents a key mechanism of breast cancer resistance to CDK4/6i (Alvarez-Fernandez and Malumbres, 2020). Inhibition of the CDK2 bypass mechanism may therefore block proliferation of CDK4/6i-resistant tumors. Furthermore, the combination of CDK 4/6 (or CDK4 selective) inhibitors with CDK2 may lead to more durable responses in HR+ breast cancer, overcoming primary and acquired resistance to CDK4/6i.
Bispecific Antibody-Drug Conjugates (BsADCs)
Bispecific antibody‒drug conjugates (BsADCs) are designed to bind to two different co-expressed tumor target antigens while delivering a cytotoxic payload. The bispecific ADC design aims to enhance tumor selectivity over healthy cells, improve payload internalization by tumor cells, and overcome resistance mechanisms.
Clinical Trials
Study of AVZO-021 (ARTS-021) in Patients with Advanced Solid Tumors Phase 1/2, Status: Recruiting in the United States
AVZO-021 (ARTS-021) is being developed for the treatment of patients with solid tumors. Phase 1 is a dose-escalation phase aimed at assessing the safety and tolerability of AVZO-021 (ARTS-021) and determining the recommended Phase 2 dose. Phase 2 is a dose-expansion phase that will be conducted to assess the antitumor activity of AVZO-021 (ARTS-021) in patients with relapsed/refractory, unresectable locally advanced, or metastatic solid tumors. Learn more.
For questions about our clinical trials, please contact us via email at clinicaltrials@avenzotx.com.



